Abstract
Introduction
Signaling by the small GTPase Rap1B is critical for the rapid inside-out activation of platelet integrins, a prerequisite for platelet aggregation and hemostatic plug formation at sites of vascular injury. The guanine nucleotide exchange factor (GEF), CalDAG-GEFI (CDGI), is the predominant Rap-GEF in platelets. Cell biological studies demonstrated that agonist receptor-mediated increases in cytosolic calcium are necessary for CDGI activity, but it is currently not known if and how calcium affects CDGI activity.
Methods
Recombinant CDGI (1-551) and Rap1B (1-181) proteins were purified to test nucleotide exchange activity in a fluorescence-based functional assay. Hydrogen-deuterium exchange mass spectrometry was utilized to measure dynamic differences between the active and inactive forms of CDGI.
Results
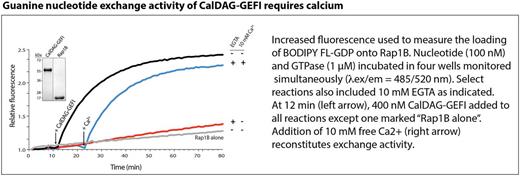
Nucleotide exchange activity of CDGI was strongly diminished by substitution of calcium-binding residues E450A or E479A within EF hand 1 and EF hand 2, respectively. Addition of exogenous calcium restored catalytic activity in CDGI (E479A) but not CDGI (E450A) or CDGI (E450A+E479A). Calcium-bound active CDGI exhibited faster deuterium exchange compared to the calcium-free inactive form, in particular for residues within the EF hands and a potential autoinhibitory linker region connecting the EF hand domains and the Cdc25 catalytic domain.
Consistent with regulation by an autoinhibitory linker, catalytic activity was fully restored in CDGI (E450A+E479A) by a gain-of-function substitution in valine 406.
Conclusions
These findings propose a model where calcium-induced conformational changes within the EF hands and autoinhibitory linker region are necessary for Rap1B to gain access to the catalytic binding pocket in the Cdc25 domain of CDGI. These findings will be important in our understanding of how mutations in patients impair CDGI function and in efforts to design inhibitors of CDGI signaling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal